Chiral separation
Many organic molecules are chiral, i.e. they can occur in a right-handed and left-handed form, which are non-superimposable mirror images of each other. The pharmaceutical industry often produces chemicals as racemic mixtures, while for many applications chirally pure compounds are needed. An important approach to arrive at chirally pure compounds is via crystallization. One method (discovered in Groningen in 1999) is Dutch resolution, which involves the use of a family of resolving agents in order to form diastereomeric crystals. In collaboration with several partners, we have shown that nucleation inhibition plays a decisive role in this process.
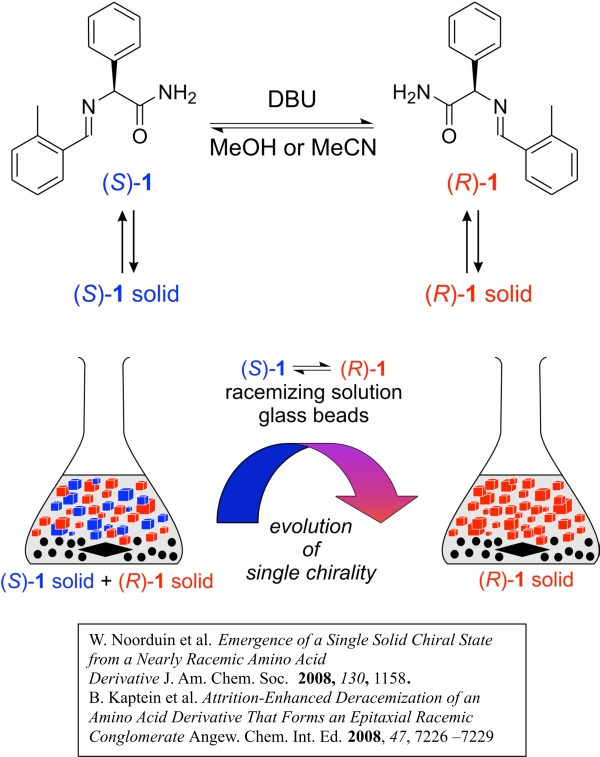
We recently discovered a new resolution method in which grinding a mixture of left- and right-handed crystals in contact with a solution containing a racemizing agent leads to conversion of all solid material to a single chirality. This conversion is explained by interplay of attrition, leading to smaller crystals, and Ostwald ripening, giving larger ones. This method is highly promising for application in industry, but is also an attractive option for explaining the single handedness of molecules in living nature.